WHY SET UP A RARE DISEASE PATIENT REGISTRY
International interoperable patient registries are particularly important for rare diseases. They bring together a small patient population which can be used to initially complete a natural history of the disease and then engage with academia and pharma companies. Disease Registry Software and Patient Registry software, ultimately, improve the quality of life of patients.
“Creating a registry of patients is the single most valuable action a rare disease community can take.” said David Meeker, President & CEO of Genzyme. “The registry provides critical disease knowledge which makes that disease easier to study, increasing the probability a treatment can be developed.”
From patient registry software to a Rare Disease Management System
The basic function of a rare disease registry is to collect the health factors of a population affected by a specific disease. They range from being very basic spreadsheets to a more complex disease specific Rare Disease Registry or Rare Disease Management System that record all patients encounters and give a complete longitudinal view of the disease.
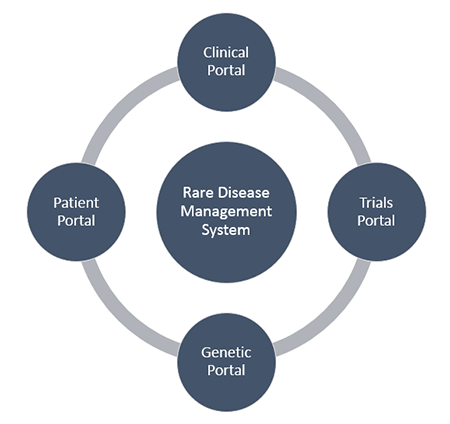
Considerations when selecting patient registry software should include; information security, data protection, streamlined consent for drug trials, integration capabilities with external clinical software and registries, pedigree and family tree considerations and patient access through patient portals.
8 steps to a rare disease registry
The eight steps outlined below shows how a patient organisation can raise their visibility & garner interest from both academics and pharmaceuticals for research into their disease.

Do these challenges sound familiar?
- Silos of information and non-interoperable datasets - Most rare disease registries in Europe are designed around national rare disease plans and are limited to that country. They have limited funding, insufficient income and therefore limited sustainability. National registries have produced silos of information, with non-interoperable datasets that are difficult to share with other stakeholders.
- Most registries are not robust enough to meet European Medicines Agency (EMA) requirements for Phase IV post marketing authorisation observational requirements, which means the time taken to bring new drugs to patients is increased.
- Most registry vendors have limited domain knowledge and are reliant on patient organisations to provide direction.
- Many registries failed to enrol sufficient patients because they are of no immediate benefit to patients or clinicians.
- Registries that are unable to handle common exceptions such as patients moving from one centre to another lead to ramifications for security, consent and reporting.
All rare disease patient registries are not the same
Patient registries fall into three main categories or levels:
- Level 1: Basic population registry with diagnosis and demographic information.
- Level 2: Population registry with an annual summary showing progression of key quantitative elements of the disease.
- Level 3: A population registry with encounter data for all health care interactions, as required for ERNs and clinical trials. Our rare disease management system, VitroRareDis includes Level 3 branching registry and is based on a e-health patient centric view of rare disease.
Patient portals
In a patient-centred healthcare model, patients are encouraged to play a greater role in tracking their health. The registry should offer secure online access to allow patients to review data such as lab results.
- Using PROMs (Patient Recorded Outcome Measures) via a patient portal, patients can update their own information.
- Addressing the growth of mHealth, registries will in future need to import and analyse data from a variety of smart phone apps.
- Vitro’s approach is to separate the patient owned data elements from the clinician owned data elements.